 Through the 300 million years that trilobites existed,
prior to their extinction
in the Permian, there were many opportunities for diversification of form,
starting from the presumed primitive morphology exemplified by a
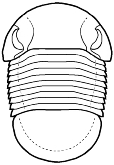
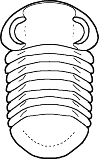
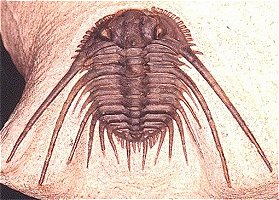
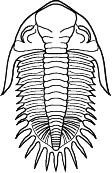
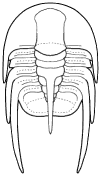
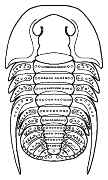
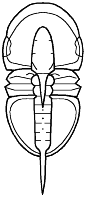
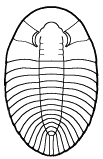
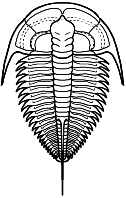
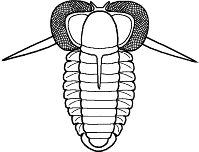
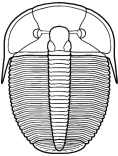
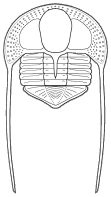
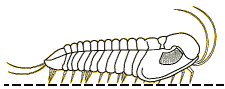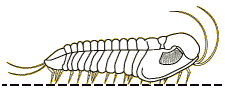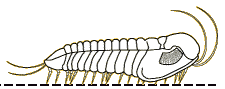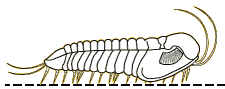
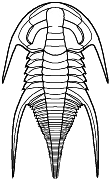
species such as Redlichia (left). This typical primitive morphotype
had a small pygidium, well developed eye ridges, a simple, lobed glabella,
several thoracic segments, and a rather flattened body form. The first trilobites were characterized by this primitive
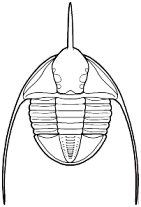
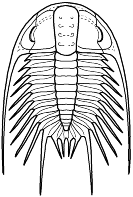
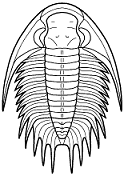
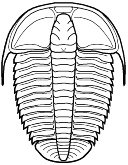
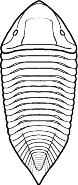
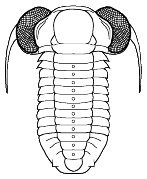
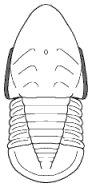
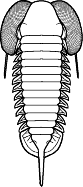
form. Among the over 20,000 species of described trilobites there are species
in which aspects of morphology have diverged greatly from the primitive state.
These are discussed in Fortey & Owens 1997 (see citations below). Thoracic
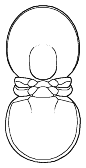
segments were reduced to as few as two or increased to
over 100, overall body shape was greatly elongated in
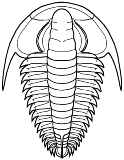
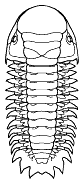
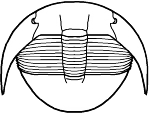
some, or rendered transverse (widened) in others. Examples are shown
below:
Through the 300 million years that trilobites existed,
prior to their extinction
in the Permian, there were many opportunities for diversification of form,
starting from the presumed primitive morphology exemplified by a
species such as Redlichia (left). This typical primitive morphotype
had a small pygidium, well developed eye ridges, a simple, lobed glabella,
several thoracic segments, and a rather flattened body form. The first trilobites were characterized by this primitive
form. Among the over 20,000 species of described trilobites there are species
in which aspects of morphology have diverged greatly from the primitive state.
These are discussed in Fortey & Owens 1997 (see citations below). Thoracic
segments were reduced to as few as two or increased to
over 100, overall body shape was greatly elongated in
some, or rendered transverse (widened) in others. Examples are shown
below:
|
|
|
|
|
||
| Related topics: Trilobite Origins Trilobite Extinction |
|
|
|
|
|
|
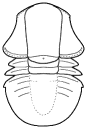
Cheirurina Phacopida |
Illaenina Corynexochida |
Emuelloidea Redlichiida |
Corynexochina Corynexochida |
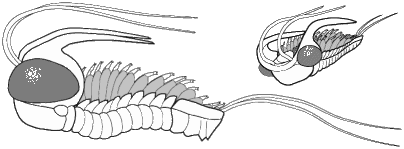
Shapes and furrow patterns of the glabella, and the shape and placement of eyes and eye ridges of course also ranged widely. An analysis of morphological diversity of trilobite forms showed that increasing from the Cambrian, there was a peak in morphological diversity in the Ordovician (which parallels a peak in overall diversity of trilobites families) that decreased only as overall trilobite diversity decreased toward their extinction in the late Permian.
Within this diversification, there were a number of evolutionary trends in morphology that developed in unrelated clades, creating homeomorphy (attainment of similar forms in unrelated groups). These homeomorphic trends, such as effacement, increased spinosity, reduction in body size, streamline shape, and loss of eyes, can not be reliably or consistently used to assess higher systematic relationships. Instead, these features can tell us about selective pressures on trilobites and how similar solutions were derived in parallel by different evolutionary lineages. Each of these is discussed below, and examples are given from different orders.