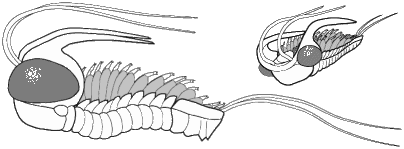
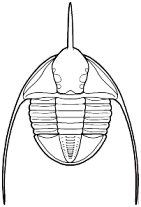
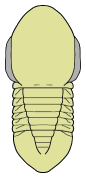
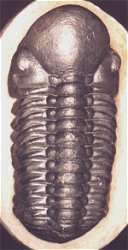
Olenoides sp. (Corynexochida) This formidable looking trilobite was probably an active predator of benthic invertebrates such as worms.
We know about the limbs of Olenoides and other trilobites thanks to the remarkable preservation of the Burgess Shale specimens, and those of other Konservat-Lagerstatten (very well conserved fossil deposits)

 Predator-scavengers
Predator-scavengers
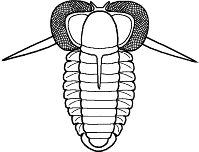
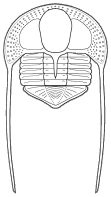
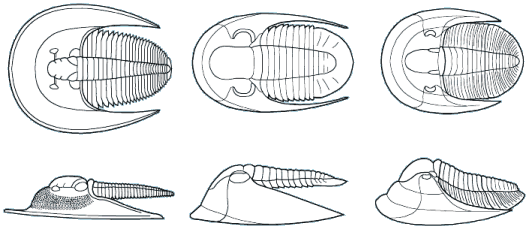
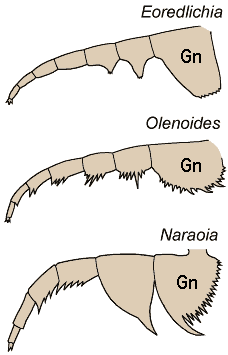
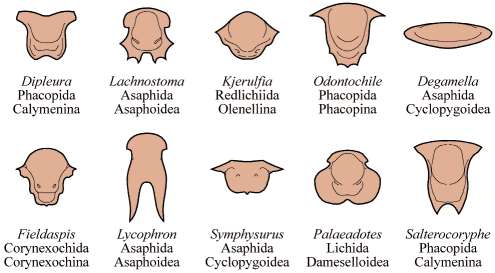
The majority of early trilobites are thought to have been predators of benthic invertebrates, such as worms, and Cambrian trilobites such as Olenoides (far left) often bore expanded and spiny gnathobases (labeled "Gn" in the examples at left). Fossilized trilobite trails sometimes stop when they intersect worm burrows (suggesting that the trilobite was hunting for worms, and stopped to eat when it found one in its burrow). Presumably the worm was extracted, subdued and crushed or torn apart with the leg spines and strong gnathobases, then passed forward between the legs to the anterior mouth, where last processing was done against the hypostome platform before ingestion. In crustaceans and insects, all of these functions are served by specialized anterior mouthparts on the head of the animal for processing food before ingestion. However, in trilobites, most of the processing occurred in the longitudinal medial groove between the limbs, with their repeated pairs of gnathobases, meaning that the "mouthparts" of a trilobite occupied the length of its underbody, rather than being primarily anterior. Another piece of inference on the predatory nature of early trilobites can be gained from looking at the relatives of trilobites. The sister-taxa of trilobites, such a Naraoids, also included predators (see the fang-bearing gnathobase of Naraoia compacta above, for example). Predatory trilobites, argued Fortey and Owens, would need to have conterminant hypostomes (firmly attached to the frontal doublure), essentially stabilizing the hypostome against the cephalic exoskeleton for aid in processing prey. It is interesting to note that there is a considerable variation in the size and shape of conterminant hypostoma, suggesting there might have been a great deal of trophic specialization. Below are a few examples of this variation:
©2000 by S. M. Gon III created using Macromedia Freehand
They also suggested that species with inflated glabellae (such as Phacopina, Proetoidea, etc.) might be considered predatory, with the large glabella housing a sizable digestive chamber for initial processing of large chunks of prey (vs bits of detritus). As a final note, they suggested that larger species of trilobites were very likely predatory, including many species of Asaphids, Phacopids, and Redlichiids.
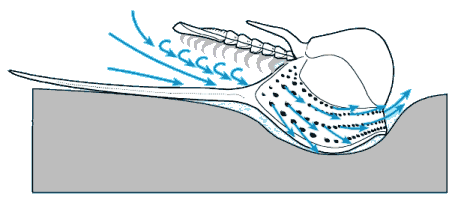
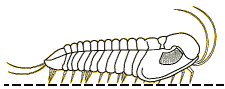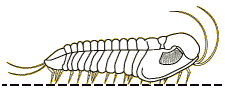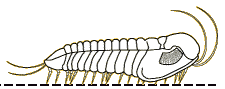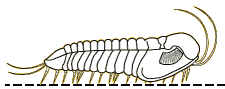
Finally, since there are few depictions of trilobites eating anything, here is my reconstruction of a large Olenoides serratus, subduing a small Ottoia (a priapulid worm) that it has just pulled from its shallow burrow. Its spiny limbs pin the hapless worm to its ventral midline, where its large gnathobases stab and tear at the worm's tough outer epidermis. Once subdued, the gnathobases will tear the worm apart and feed chunks into the mouth.
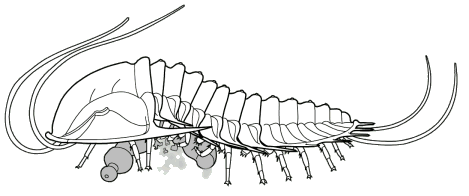
Olenoides serratus: terror of the Burgess
mudflats
©2000 by S. M. Gon III created using Macromedia Freehand
 Trilobite Feeding Habits
Trilobite Feeding Habits

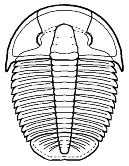
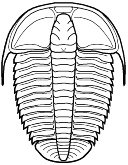

 Among the Ptychopariida, the Olenina include a number of species
that were known to occupy deep benthic, nearly anoxic substrates,
high in sulphur compounds. Modern crustaceans in those situations (as at
mid oceanic ridges and thermal vents) often live in a symbiotic relationship
with sulphur-eating bacteria that are housed in the gill
structures. Fortey suggested that this relationship may have originated
with olenid trilobites in the Palaeozic. He cites the very wide thoraxic
pleurae of many olenimorphs, increased numbers of thoracic segments (both
traits providing ample gill surface for symbionts), and a reduced hypostome,
so small that it suggests that much of the nutrient requirements for these
trilobites were not being processed and ingested, but absorbed through the
gills from symbiotic bacterial colonies there. Olenids are known for their
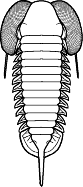
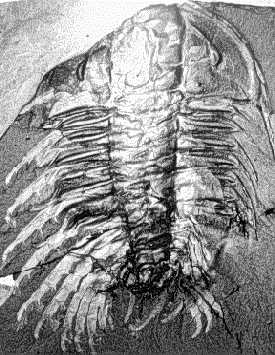
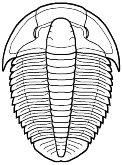
well-developed gill structures, as in this image of Triarthrus.
Among the Ptychopariida, the Olenina include a number of species
that were known to occupy deep benthic, nearly anoxic substrates,
high in sulphur compounds. Modern crustaceans in those situations (as at
mid oceanic ridges and thermal vents) often live in a symbiotic relationship
with sulphur-eating bacteria that are housed in the gill
structures. Fortey suggested that this relationship may have originated
with olenid trilobites in the Palaeozic. He cites the very wide thoraxic
pleurae of many olenimorphs, increased numbers of thoracic segments (both
traits providing ample gill surface for symbionts), and a reduced hypostome,
so small that it suggests that much of the nutrient requirements for these
trilobites were not being processed and ingested, but absorbed through the
gills from symbiotic bacterial colonies there. Olenids are known for their
well-developed gill structures, as in this image of Triarthrus.