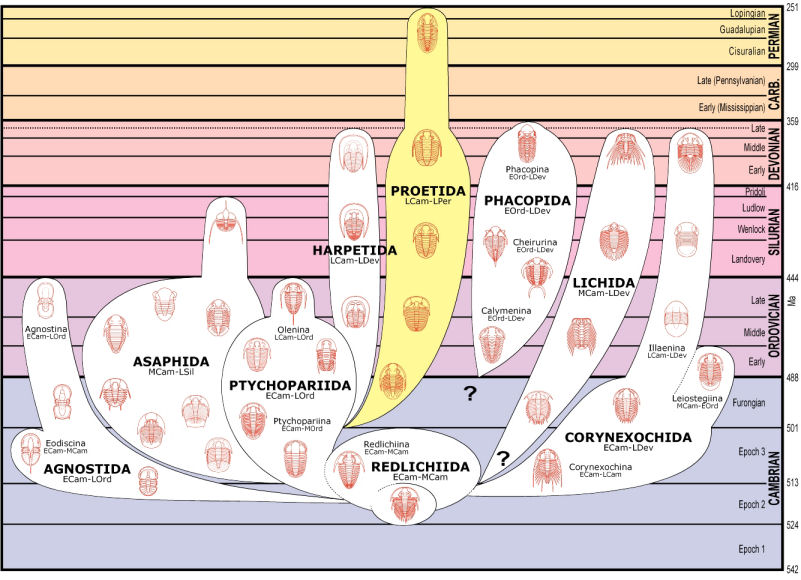
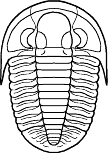
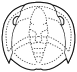
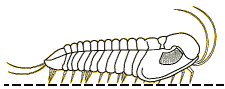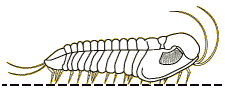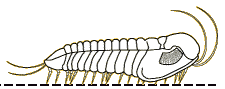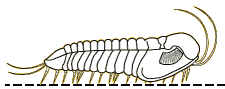
Phillipsia
.
.
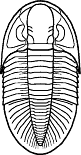
Cornuproetus

.
.
Phaetonellus
|
Superfamily Proetoidea
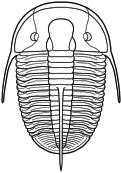
Cephalon: opisthoparian sutures, glabella
tapering or inverse pyriform, mostly suboval, with 3-4
pairs of lateral furrows, sometimes indistinct (e.g.,
Proetinae), or glabella long, expanding forward to
anterior border furrow or beyond, with lateral
preoccipital lobes present (e.g., Phillipsiinae); eyes,
when present, typically holochroal, convex; fixigenae
narrow, librigenae broad (except in blind species);
hypostoma typically natant, but secondarily conterminent
in advanced Proetidae; genal angle spined or blunt
rounded.
Thorax: 7-10 segments, typically 10 in Proetinae,
9 in Phillipsiinae, pleurae with furrows, tips blunt or
spined.
Pygidium: from small and hemispherical, with few
segments (e.g., Proetinae), to long, parabolic, with up
to 33 axial segments (e.g., Phillipsiinae); margin
typically smooth, but some with terminal axial spine or
pleural spines.
Other: anaprotaspides of Proetoidea distinct
from those of Aulacopleuroidea and Bathyuroidea
(Lerosey-Aubril, R., & R. Feist (2005) and part of
suggested basis for splitting this order into two
(Aulacopleurida & Proetida). Difficulties in finding
consistent characters diagnostic of adult Proetoidea are
discussed in greater length in "Additional Notes" below.
Families: Proetidae (subfamilies Proetinae &
Phillipsiinae -- alternately Phillipsiidae treated as
separate family, see "Additional Notes" below),
Tropidocoryphidae
Genera: Proetidae: Aayernenaytcheia,
Acanthophillipsia, Aceroproetus, Acropyge,
Alaskalethe, Altajaspis, Ameropiltonia, Ameura,
Ampulliglabella, Anambon, Anglibole, Angustibole,
Anisopyge, Anujaspis, Appendicysta, Aprathia,
Archaeocoryphe, Archegonus (=Cylindraspis),
Ascetopeltis, Astroproetus (=Clypoproetus;
=Enodiproetus; =Sibiroproetus), Australokaskia,
Bailielloides, Bapingaspis, Basidechenella, Bedicella,
Beleckella, Belgibole, Benesovella, Bitumulina,
Blodgettia, Bohemiproetus, Bolivicrania,
Boliviproetus, Bollandia, Bonnaspidella, Borealia,
Brevibole, Breviphillipsia, Burgesina, Calybole,
Camsellia, Capricornia, Carbonocoryphe,
Carbonoproetus, Carlopsia, Carniphillipsia,
Ceratoproetus, Chauffouraspis, Chaunoproetoides,
Chaunoproetus (=Carnicia), Chiides, Chiops,
Chlupacula, Chuanqianoproetus, Clavibole, Combewoodia,
Comptonaspis, Coniproetus, Conophillipsia,
Constantina, Craspedops, Crassibole, Crassiproetus,
Cummingella, Cyphinioides, Cyphoproetus,
Cyrtodechenella, Cyrtoproetus, Cyrtosymbole,
Cystispina, Daihuaia, Dayinaspis, Dechenella
(/Eudechenella), Dechenelloides, Dechenellurus,
Deinoproetus, Delaria, Deltadechenella, Diabole,
Diacoryphe, Ditomopyge (=Cyphinium; =Permoproetus;
=Neophillipsia), Doublatia, Drevermannia, Dudu,
Dushania, Effops, Ejinoproetus, Elegenodechenella,
Elimaproetus, Elliptophillipsia, Endops, Ensecoryphe,
Eocyphinium, Eocyrtosymbole, Eodrevermannia,
Eomicrophillipsia, Eopalpebralia, Eosoproetus,
Eowinterbergia, Erbenaspis, Erbenites, Evagena,
Exochops, Flexidechenella, Formonia, Francenaspis,
Franconicabole, Frithjofia, Fuscinipyge, Ganinella,
Gapeevella, Geigibole, Georhithronella, Gerastos
(=Dohmiella; =Kegeliella), Gitarra, Globusia,
Globusiella, Globusoidea, Gomiites, Gracemerea,
Griffithidella, Griffithides, Grossoproetus, Haasia,
Hamiroproetus, Hassiabole, Hedstroemia (=Milesdavis;
=Pachyproetus), Helioproetus, Helmutia, Helokybe,
Hentigia, Hesslerides, Hildaphillipsia, Humeia,
Humilogriffithides, Hunanoproetus, Hypaproetus,
Iranaspidion, Jimbokranion, Jinia, Karginella, Kaskia,
Kathwaia, Kerpenella, Khalfinella, Kollarcephalus,
Kolymoproetus, Kosovoproetus, Krambedrysia, Kulmiella,
Kulmogriffithides, Lacunoporaspis, Laevibole,
Langgonbole, Latibole, Latiglobusia, Latiproetus,
Lauchellum, Lichanocoryphe, Linguaphillipsia, Liobole,
Liobolina, Longilobus, Longiproetus, Lophiokephalion,
Lugalella, Luojiashania, Macrobole, Mahaiella,
Malayaproetus, Malchi, Mannopyge, Megaproetus,
Menorcaspis, Merebolina, Metaphillipsia,
Microphillipsia, Microspatulina, Mirabole,
Monodechenella, Moravocoryphe, Moschoglossis,
Myoproetus, Namuraspis, Neogriffithides
(=Siciliproetus), Neokaskia, Neoproetus, Nipponaspis,
Nitidocare, Nodiphillipsia, Novoameura, Nunnaspis,
Oehlertaspis (/Oehlertia), Oidalaproetus,
Orbitoproetus, Ormistonaspis, Omlistonia,
Ormistoniella, Osmolskia, Otodechenella, Paladin
(=Weberides), Palaeophillipsia, Paleodechenella,
Palpebralia, Panibole (=Proliobole),
Parachaunoproetus, Paradechenella, Parafrithjofia,
Paraglobusia, Paragriffithides, Paramirabole,
Parangustibole, Parapalpebralia, Paraphillipsia,
Paraproetus, Parawarburgella, Particeps, Parvidumus,
Paryfenus (/Colymbus), Pedinocoryphe,
Pedinodechenella, Perexigupyge, Perliproetus,
Phillibole, Phillibolina, Philliboloides
(/Phillibolina), Phillipsia, Phyllaspis, Piltonia,
Planilobus, Planokaskia, Plesiowensus, Podoliproetus,
Pontipalpebralia, Praedechenella, Pragoproetus,
Prantlia (=Malvernocare), Prodiacoryphe,
Proetocephalus, Proetus (/Euproetus; /Aeonia;
=Devonoproetus; =Falcatoproetus; =Forbesia;
=Scotoproetus; =Trigonaspis), Pseudobollandia,
Pseudocyrtosymbole, Pseudodechenella
(=Arcticormistonia), Pseudodudu, Pseudogerastos,
Pseudophillipsia, Pseudoproetus, Pseudosilesiops,
Pseudospatulina, Pseudowaribole, Pudoproetus
(=Zhifangia), Pulcherproetus, Pusillabole,
Raerinproetus, Reediella, Rhenocynproetus,
Rhenogriffides, Richterella, Rosehillia, Rugulites
(/Podolites), Ryckholtia, Schaderthalaspis,
Schizophillipsia, Schizoproetina, Schizoproetoides,
Schizoproetus, Serniproetus, Sevillia, Silesiops,
Simaproetus, Sinobole, Sinocyrtoproetus, Sinopaladin,
Sinoproetus, Sinosymbole, Skemmatocare, Skemmatopyge,
Spatulina, Spergenaspis, Spinibole, Spinibolops,
Struveproetus, Sulcubole, Tawstockia, Taynaella,
Tcherkesovia, Tetinia, Thaiaspella, Thaiaspis,
Thalabaria, Thebanaspis, Thigriffides, Timoraspis,
Triproetus, Tropiconiproetus, Tropidocare,
Tschernyschewiella (/Schmidtia), Typhloproetus,
Unguliproetus, Vandergrachtia, Vidria, Vittaella,
Wagnerispina, Waideggula, Waigatchella, Warburgella
(/Holometopus; =Owensella), Waribole, Weania,
Weberiphillipsia (=Spinolimbella), Westropia,
Weyeraspis, Winiskia, Winterbergia, Witryides,
Xenadoche, Xenoboloides, Xenocybe, Xenodechenella,
Xiangzhongella, Xiushuiproetus, Yanshanaspis,
Yichangaspis, Yishanaspis, Zhegangula,
Zhejiangoproetus.
Tropidocoryphidae: Alberticoryphe,
Astycoryphe, Bojocoryphe, Buchiproetus, Centriproetus,
Cornuproetus, Cyrtosymboloides, Dalarnepeltis,
Dalejeproetus, Decoroproetus (=Ogmocnemis; =Proetidella;
=Warburgaspis), Denemarkia, Diademaproetus, Dipharangus,
Eopiriproetus. Erbenicoryphe, Eremiproetus
(=Natatoraspis; =Dufresnoyiproetus), Gracilocoryphe,
Gruetia, Guilinaspis, Hollardia, Ignoproetus,
Interproetus, Koneprusites, Krohbole, Lardeuxia,
Laticoryphe, Lepidoproetus, Linguaproetus, Lodenicia,
Longicoryphe, Macroblepharum, Miriproetus, Nagaproetus,
Paraeremiproetus, Paralardeuxia, Paralepidoproetus,
Parvigena, Perakaspis, Phaetonellus, Phaseolops,
Piriproetoides, Piriproetus, Pribylia, Prionopeltis
(/Phaetonides/Phaeton), Prodrevermannia, Proetina,
Proetopeltis, Pterocoryphe, Pteroparia, Quadratoproetus,
Rabuloproetus, Ranunculoproetus, Remacutanger,
Richteraspis, Rokycanocoryphe, Sculptoproetus,
Slimanella, Spinoproetus, Stenoblepharum (=Viruanaspis),
Tafilaltaspis, Tropicoryphe, Tropidocoryphe,
Vicinoproetus (/Vicinopeltis), Voigtaspis, Wolayella,
Xiphogonium (=Trautensteinproetus), Zetaproetus.
|
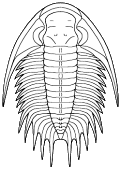
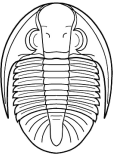
Bathyurus
.
.
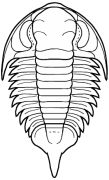
Acidiphorus
.
.
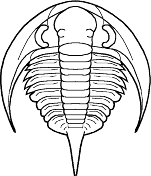
Platyantyx
|
Superfamily Bathyuroidea
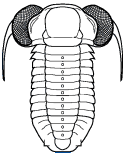
Cephalon: convex, with distinct border of
varying width, opisthoparian sutures; glabella well
defined, subparallel or gently expanding/tapering
forward, with 3 or fewer lateral furrows, sometimes
faint or absent; eyes medium to large, placed opposite
or behind center of glabella; hypostoma typically
natant, but secondarily conterminent in advanced
Bathyuridae; genal spines typically present, sometimes
broad.
Thorax: 8-12 segments, pleurae typically with
distinct furrows.
Pygidium: subisopygous to micropygous, gently to
moderately convex, no border furrow, axis of varying
length (absent in Celmidae), sometimes prolonged into
post-axial ridge or axial spine, often 3 axial rings +
terminal; exoskeleton sometimes ornamented variously
with pits, tubercles, small spines, or terraces.
Families: Bathyuridae, Dimeropygidae (including
Celmidae), Holotrachelidae, Hystricuridae, Raymondinidae
(including Glaphuridae), Telephinidae, Toernquistiidae.
Genera: Bathyuridae: Acidiphorus
(=Goniotelina; =Goniotelus/Goniurus), Aksuaspis,
Bathyurellus, Bathyurus, Benthamaspis (=Oculomagnus),
Bolbocephalus, Catochia, Ceratopeltis,
Eleutherocentrus, Ermanella, Gignopeltis,
Grinnelaspis, (/Actinopeltis Poulsen),
Hadrohybus, Jeffersonia (=Bathyurina), Licnocephala
(=Domina), Lutesvillia, Madaraspis, Peltabellia
(=Biolgina), Petigurus, Platyantyx, ?Proscharyia,
Psephosthenaspis (=Aponileus; =Ludvigsenella),
Pseudoolenoides, Punka, Rananasus, Randaynia,
Raymondites, Sinobathyurus, Strigigenalis, Uromystrum.
Dimeropygidae (incl. Celmidae): Celmus
(=Crotalurus; =Ischyrophyma), Dimeropyge (/Haploconus),
Dimeropygiella, Glaphurella, Ischyrotoma,
Pseudohystricurus.
Holotrachelidae: Holotrachelus,
Kinderlania.
Hystricuridae: Amblycranium,
Etheridgaspis, Flectihystricurus, Genalaticurus,
Glabretina, Guizhouhystricurus, Hillyardina
(=Metabowmania), Hintzecurus, ?Holubaspis (/Holubia),
Hyperbolochilus, Hystricurus (=Vermilionites),
Ibexicurus, Lavadamia, Nyaya, Omuliovia, Pachycranium,
Paenebeltella, Parahystricurus, Paraplethopeltis,
Politicurus, Psalikilopsis, Psalikilus, Rollia,
Rossicurus, Tanybregma, ?Taoyuania (=Batyraspis),
Tasmanaspis, Tersella.
Raymondinidae (incl. Glaphuridae): Glaphurina,
Glaphurus, Raymondina (=Raymondia), Tagazella,
Varanella.
Telephinidae: Carolinites
(=Dimastocephalus; =Keidelia; =Tafnaspis), Fialoides,
Goniophrys, Oopsites, Opipeuterella (=Ompheter;
=Opipeuter), Paraphorocephala, Phorocephala
(=Carrickia), ?Pyraustocranium, Telephina (=Telephus),
Telephops.
Toernquistiidae: Chomatopyge,
Mesotaphraspis, Toernquistia (=Paratoernquistia),
?Toernquistina. |