The Anomalocaris Homepage
page
last revised 27 June 2008 by Sam Gon III------
The Anomalocarid Bauplan
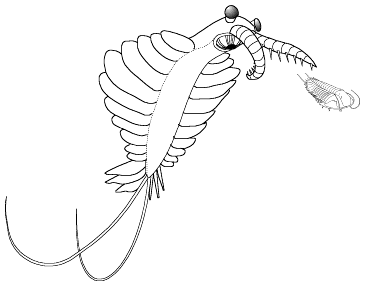
.
. Anomalocaris saron pursues a swimming
trilobite. |
The studies of the mid
to late 1990s have presented us with intriguing revelations, and the most
complete fossils of anomalocaridids. The level of current research and
discovery is at its most promising since Whittington and Briggs (1982, 1985)
pieced together the first reasonable reconstruction of Anomalocaris.
The anomalocaridid bauplan (body plan)
All anomalocaridids are bilaterally symmetrical,
bear a pair of anterior segmented appendages, these often sclerotized
and armed with spines, a ventral anterior mouth is comprised of tooth-bearing
sclerotized wedge-shaped segments arranged in a ring around a central
aperture. Jaws, jaw segments, and anterior claws are more often preserved
than the rest of the animal, suggesting that these parts are the most robust.
Dorsolateral prominent eyes, most often a single pair placed to either
side of the mouth, but sometimes either anterior or posterior of the mouth,
are typically set on flexible stalks of various length. An elongate, metameric
body bears numerous (typically 10+) pairs of lateral imbricating lobes,
probably of natatorial function, sometimes accompanied by repeating sets
of horizontal support rods. In the majority of species for which complete
specimens are known, there are (typically three) pairs of prominent dorsolateral
fins immediately posterior to the lateral lobes.
|
| However, the anomalocaridid
bauplan appears to be more variable than previously assumed, and efforts
should be directed towards compiling a new, complete list of diagnostic
characters for the family. A revised list of diagnostic characters could
ultimately affect the relationship between the Anomalocarididae and other
possible families within Collins' (1996) proposed Order Radiodonta and
Class Dinocarida. Both of these taxa are important in the effort to place
anomalocarids and other similar problematic metazoans (e.g. Opabinia
regalis, Kerygmachela kierkegaardi) into an eventually coherent
phylogenetic context. Collins' proposal of the class Dinocarida is not
universally accepted by others working on anomalocaridids. Chen and Zhou
(1997) place anomalocaridids at the phylum level, but without providing
a formal, comprehensive diagnosis, or cladistic analysis.
Variation in anomalocarid characters:
The 'fantail' reported in Anomalocaris
canadensis (Collins 1996), A. saron, and Amplectobelua symbrachiata
(Chen et al. 1994) presents a comparative problem. Phylogenetically, it
is certainly not homologous with a telson, and no other arthropod (except
Opabinia, itself arguably closely related to anomalocarids)
has a similar posterodorsally placed structure. It is unclear whether
the 'fantail' elements were rigidly fixed, or moveable, like the lateral
lobes. The presence of caudal furcae in the Chengjiang material is certainly
an arthropodan character, but the 'fantail' complex is a uniquely derived
feature. Part of the problem in adequately classifying the anomalocarids
is that they seem to have developed a significant amount of derived features
after diverging from basal arthropods.
Recent studies dramatically emphasize the variations
found in the anterior grasping appendages among anomalocaridid genera. Described
examples are the relatively stout crushing claws of Anomalocaris canadensis
(Collins 1996); the fearsome impaling claws of Amplectobelua symbrachiata;
and the long, slender claws of Anomalocaris saron (Chen et al. 1994;
Hou et al. 1995). In contrast, the feeding appendages of Laggania cambria
(Briggs' "Appendage F") with its long, delicate rake spines, seem more appropriate
for a planktonic sweep-feeder than for a rapacious predator of armored arthropods
(see discussion by Dzik and Lendzion 1988, and Nedin 1995). Similar appendages
on other anomalocarid species (such as those of Anomalocaris briggsi
from the Emu Bay Shale, Australia, known only from appendages with
extensive comb rows on all but the first podomere endites) suggest that both
predator and planktivore guilds may have been occupied by anomalocaridids
(see Nedin 1995).
|
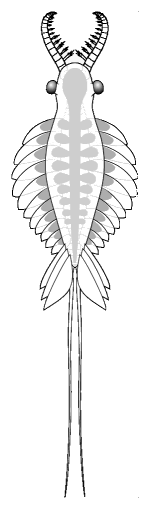
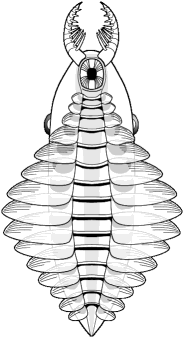
dorsal view |
anterior appendages
dorsolateral eyes
circular ventral mouth
elongate metameric body
numerous lateral lobes
dorsolateral fantail
paired furcae
Anomalocaris
saron
Chengjiang, China |
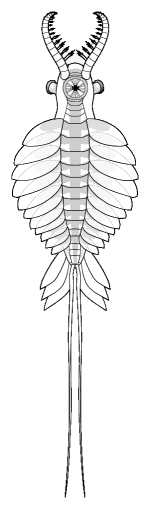
ventral view |
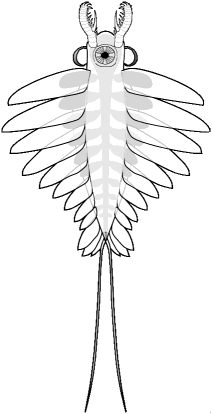
Some variations in the Anomalocaridid
Bauplan
Anomalocaris canadensis
Burgess Shale, Canada
|
Anomalocaris saron
Chengjiang, China

|
Laggania cambria
Burgess Shale, Canada
|
Amplectobelua symbrachiata
Chengjiang, China
|
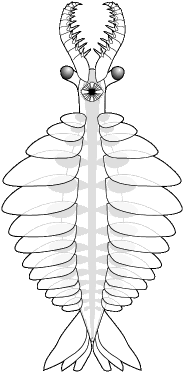
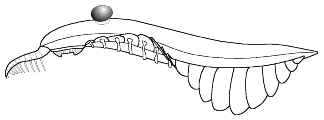
| Eyes anterior of mouth,
narrow anterior body, heavily armed anterior appendages, strong fantail,
lacking furcae, |
Eyes lateral to mouth,
largest mouth segments arranged diagonally, heavily armed anterior appendages,
strong fantail, with furcae |
Eyes far posterior of
mouth, broad anterior body, weakly armed anterior appendages, ventral,
transverse lobe support rods, lacking fantail and furcae |
Eyes large, lateral
to mouth, impaling basal spines on anterior appendages, fantail with furcae |
Some anomalocaridid morphotypes also show an increase
in their degrees of tagmosis (body segmentation) and sclerotization (hardened
exoskeleton), while others show the opposite. For example, Collins' complete
specimens of Anomalocaris canadensis confirm that there was no
evidence of trunk annulation or external segmentation preserved in this
species. At least one purported anomalocaridid (Parapeytoia) had
pairs of segmented gnathobasic legs associated with each trunk segment.
Swimming lobes are thought to be expanded exopod elements of biramous limbs.
Certain groups of anomalocaridids ceased developing biramous trunk appendages
in favor of retaining only the lateral lobes. Interpreted in an arthropod
context, this change translates into the loss of the endopod and the retention
of the exopod. Those anomalocarids that retain segmentation, sclerotization,
and endopods most strongly demonstrate arthropod origins, while taxa showing
loss of endopods, poor sclerotization, and ambiguous tagmata demonstrate
the derived extreme.
Organization of major grades of anomalocarids:
1. Sclerotized mouthparts and grasping appendages;
body is 'naked', smooth-surfaced without visible trunk annulation or external
segmentation; no apparent external gill-like structures; no body ornamentation
or raised features of any kind; 13 pairs of lateral lobes; no jointed
trunk appendages, and posterodorsal 'fantail' finlets arranged en echelon.
(after Collins 1996)
Example: Anomalocaris canadensis |
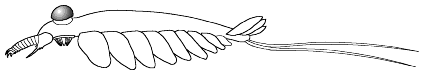
2. Sclerotized mouthparts and grasping appendages;
diagonal striations on the lateral lobes (interpreted as veins by some
authors); setae-like structures present on lateral lobes; two exsaggital
ventral rows of serially-repeated, nodular structures; no confirmed trunk
annulation or external segmentation; no jointed trunk appendages; tail furcae
present; and posterodorsal 'fantail' finlets arranged en echelon. (After
Chen et al. 1994; Hou et al. 1995).
Examples: Anomalocaris saron, Amplectobelua
symbrachiata (shown here) |
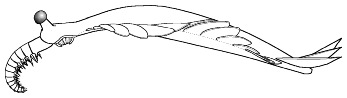
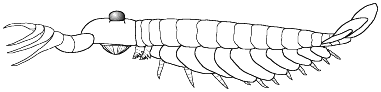
3. Sclerotized mouthparts and grasping appendages;
body is 'naked', smooth-surfaced without visible trunk annulation or external
segmentation; no apparent external gill-like structures; ventral, transverse,
lateral lobe support 'rods' present. 14 pairs of lateral lobes; no jointed
trunk appendages, evidence equivocal concerning presence of striations
on the entire ventral surface of the lateral lobes; caudal end of body tapers
to blunt extremity. No fantail or furcae. (after Collins 1996)
Example: Laggania cambria |
4. Significantly sclerotized body including
median sternites, ?dorsal lanceolate scales, gnathobasic biramous trunk
appendages, grasping appendages; diagonal striations present on lateral
lobes (after Hou et al. 1995). Other forms awaiting description by Ramskold
(1995) could fall into this group, further expanding its diagnostic criteria.
However, Chen et al 2004 suggests Parapeytoia may be more aligned
with 'great-appendage' species such as Yohoia, Haikoucaris, Leanchoilia,
Jiangfengia, and Fortiforceps than with anomalocaridids.
See page on Parapeytoia
for further discussion.
Examples: Parapeytoia yunnanensis
(shown here), Cucumericrus decoratus |
[no image available]
5. Anterior tagmata formed of several carapace-like
components bearing two sheathed claws on stalks. Below the carapaces,
the jaws have an inner set of teeth, and are surrounded by pair of claws;
trunk has 11 segments and a tail; gnathobasic, biramous trunk appendages
(after Collins 1992, 1996).
Example: Hurdia sp., Proboscicaris
sp. |
| 6. Uncertain: Cassubia infercambriensis,
Anomalocaris briggsi, and Anomalocaris pennsylvanica
(incl. cf. pennsylvanica) are indeterminate forms based on grasping
appendages only. Amiella ornata is a nomen dubium (Hou and
Bergstrom 1997). The named species assigned to Hurdia (e.g. H.
dentata, H. triangulata and H. victoria) and Proboscicaris
(e.g. P. agnosta, P. ingens and P. obtusa) should all be regarded
as nomina dubia, pending a full description of these genera. |
Anomalocaridid References (post 2000 references highlighted yellow)
Babcock, L.E. 1993. Trilobite malformations and
the fossil record of behavioral asymmetry. Journal of Paleontology, 67,
217-229.
Babcock, L.E. and Robison, R.A. 1989. Preferences
of Palaeozoic predators. Nature, 337, 695-696.
Briggs, D.E.G. 1979. Anomalocaris, the largest
known Cambrian Arthropod. Palaeontology, 20, 631-664.
Briggs, D.E.G. 1994. Giant predators from the Cambrian
of China. Science, 264, 1283-1284.
Briggs, D.E.G. and Mount, D.J.D. 1982. The occurrence
of the giant arthropod Anomalocaris in the Lower Cambrian of Southern
California, and the overall distribution of the genus. Journal of Paleontology,
56, 1112-1118.
Briggs, D.E.G. and Whittington, H.B. 1987. The affinities
of the Cambrian animals Anomalocaris and Opabinia. Lethaia
20, 125-6.
Briggs, D.E.G., B.S. Liebermann, J.R. Hendricks, S.L. Halgedahl & R.D. Jarrard. 2008. Middle Cambrian arthropods from Utah. Journal of Paleontology 82(2):238-254.
Budd, G.E. 1998. Stem group arthropods from the Lower Cambrian Sirius Passet fauna of North Greenland. in: R.A. Forety and R.H. Thomas (eds.) Arthropod Relationships. Chapman & Hall, London.
Chen, J. Waloszek, D. & Maas, A. 2004. A new
'great-appendage' arthropod from the Lower Cambrian of China and homology
of chelicerata chelicerae and raptorial antero-ventral appendages. Lethaia
37, 3-20.
Chen, J., Ramskold, L. and Zhou Guiquing, 1994.
Evidence for monophyly and arthropod affinity of Cambrian giant predators.
Science, 264, 1304-1308.
Chen, J. and Zhou Guiquing, 1997. Biology of the
Chengjiang Fauna. Bulletin of the National Museum of Natural Science,
10, 11-105
Collins, D. 1992. Whither Anomalocaris? The
search in the Burgess Shale Continues. Abstracts, Fifth North American Paleontological
Convention, Chicago, Paleontological Society Special Publication, 6,
66.
Collins, D. 1996. The "evolution" of Anomalocaris
and its classification in the arthropod class Dinocarida (nov.) and order
Radiodonta (nov.). Journal of Paleontology, 70, 280-293.
Collins, D., Bergstrom, J. and Seilacher, A. 1991.
Chengjiang Fauna. National Geographic Research and Exploration 7(2),
238-239.
Dzik, J. and Lendzion, K. 1988. The oldest arthropods
of the East European Platform. Lethaia, 21, 29-38.
Henricksen, K.L. 1928. Critical notes upon some
Cambrian arthropods described by Charles D. Walcott. Videnskabelige Meddelelser
fra Dansk Naturhistorisk Forening: Khopenhavn, 86:1-20.
Hou Xianguang and Bergstrom, J. 1997. Arthropods
of the Lower Cambrian Chengjiang fauna, southwest China. Fossils and
Strata, 45, 1-116.
Hou Xianguang and Bergstrom, J. and Ahlberg, P.
1995. Anomalocaris and other large animals in the Lower Cambrian Chengjiang
fauna of southwest China. GFF, 117, 163-183.
Hou, X.G., R.J. Aldridge, J. Bergstrom, D.J. Siveter, & X.F. Feng. 2004. The Cambrian Fossils of Chengjiang, China. Blackwell Publishing, Oxford, U.K. 233 pp.
Liebermann, B.S. 2003. A new soft-bodied fauna: The Pioche Formation of Nevada. Journal of Paleontology 77:676-692.
Nedin, C. 1995. The Emu Bay Shale, a Lower Cambrian
fossil Lagerstätten, Kangaroo Island, South Australia. Memoirs
of the Association of Australasian Palaeontologists 18, 31-40.
Ramskold, L. 1995. From characters to clades: interpreting
Lobopodians and Anomalocaridids. 22. In Chen Junyuan, Edgecombe, G. and
Ramskold, L. (eds). International Cambrian Explosion Symposium (April,
1995, Nanjing) (Programme and Abstracts). Nanjing Institute of Geology
and Palaeontology, Academia Sinica, 48 pp.
Ramskold, L. 1997. Preservational folds simulating
tergite junctions in tegopeltid and naraoiid Arthropods. Lethaia, 29,15-20.
Ramskold, L., Chen Junyuan., Edgecombe, D. and Zhou
Guiquing, 1997. Cindarella and the arachnate clade Xandarellida(Arthropoda,
Early Cambrian) from China. Transactions of the Royal Society of Edinburgh:
Earth Sciences, 88,19- 38.
Rudkin, D.M. 1979. Healed injuries in Ogygopsis
klotzi (Trilobita) from the Middle Cambrian of British Columbia. Royal
Ontario Museum, Life Sciences Occasional Paper, 32, 1-18.
Walcott, C. D. 1911. Middle Cambrian Holothurians
and Medusae. Cambrian geology and paleontology II. Smithsonian Miscellaneous
Collections 57:41-68.
Whittington, H.B., and Briggs, D.E.G. 1982. A new
conundrum from the Middle Cambrian Burgess Shale. 573- 575. In Mamet, B.,
and Copeland, M. J. (eds). Proceedings of the Third North American Paleontological
Convention, Montreal, 2. Department of Geology, University of Montreal,
and Geological Survey of Canada, Ottawa.
Whittington, H.B., and Briggs, D.E.G. 1985. The
largest Cambrian animal, Anomalocaris, Burgess Shale, British Columbia.
Philosophical Transactions of the Royal Society of London, Series B,
309, 569-609.
Click here to view the first pair of species accounts: Anomalocaris saron & Amplectobelua symbrachtiata









